Matching (Value 5)
|
|
|
Match each item with the correct statement below. a. | acid dissociation constant (Ka) | d. | Lewis acid | b. | diprotic
acid | e. | pH | c. | hydrogen-ion
donor |
|
|
|
1.
|
acid with two ionizable protons
|
|
|
2.
|
ratio of the concentration of the dissociated to the undissociated form
|
|
|
3.
|
can accept an electron pair
|
|
|
4.
|
Brønsted-Lowry acid
|
|
|
5.
|
negative logarithm of the hydrogen ion concentration
|
Multiple Choice (Value 24)
Identify the choice that best completes the statement or answers
the question.
|
|
|
6.
|
Which of the following is a property of an acid?
a. | unreactive | c. | strong color | b. | nonelectrolyte | d. | sour taste |
|
|
|
7.
|
What is transferred between a conjugate acid-base pair?
a. | an electron | c. | a hydroxide ion | b. | a hydronium ion | d. | a proton |
|
|
|
8.
|
The formula of the hydrogen ion is often written as ____.
|
|
|
9.
|
What is the best description for a solution with a hydroxide-ion concentration
of 1 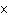 10 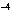 M M? a. | acidic | c. | basic | b. | neutral | d. | The answer cannot be
determined. |
|
|
|
10.
|
Which of these solutions is the most basic?
|
|
|
11.
|
A Lewis acid is a substance that can ____.
a. | donate a hydrogen ion | c. | accept a pair of electrons | b. | donate a pair of
electrons | d. | accept a hydrogen
ion |
|
|
|
12.
|
Which of these is an Arrhenius base?
|
|
|
13.
|
In the reaction of aluminum bromide with ionized sodium bromide, which compound
is the Lewis acid?
a. | aluminum bromide | c. | sodium ion | b. | bromide ion | d. | None are Lewis
acids. |
|
|
|
14.
|
In a neutral solution, the [H 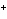 ] is ____. a. | zero | c. | 1 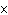 10 10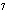 M M | b. | 10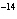 M M | d. | equal
to [OH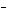 ] ] |
|
|
|
15.
|
Which compound can act as both a Brønsted-Lowry acid and a
Brønsted-Lowry base?
a. | ammonia | c. | hydrochloric acid | b. | water | d. | sodium
hydroxide |
|
|
|
16.
|
What are the acids in the following equilibrium reaction? CN 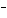 +
H 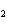 O  HCN + OH 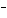
|
|
|
17.
|
Which hydroxide compound yields the lowest concentration of hydroxide ions in
aqueous solution?
a. | potassium hydroxide | c. | magnesium hydroxide | b. | calcium hydroxide | d. | sodium
hydroxide |

|
|
|
18.
|
When an acid reacts with a base, what compounds are formed?
a. | a salt and water | c. | a salt only | b. | metal oxides only | d. | water only |
|
|
|
19.
|
What type of acid is sulfuric acid?
a. | triprotic | c. | diprotic | b. | monoprotic | d. | none of the
above |
|
|
|
20.
|
In the reaction CO 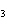 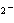 + H 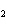 O 
HCO 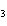 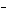 + OH 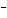 , the carbonate ion is acting as
a(n) ____. a. | Brønsted-Lowry acid | c. | Arrhenius acid | b. | Brønsted-Lowry
base | d. | Arrhenius
base |
|
|
|
21.
|
Which type of solution is one with a pH of 8?
a. | basic | b. | neutral | c. | acidic | d. | The type varies, depending on the
solution. |
|
|
|
22.
|
Which of the following reactions illustrates amphoterism?
|
|
|
23.
|
If the hydrogen ion concentration of a solution is 10 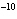 M M, is the
solution acidic, alkaline, or neutral? a. | alkaline | c. | acidic | b. | The answer cannot be
determined. | d. | neutral |
|
|
|
24.
|
What is an acid according to Arrhenius?
a. | a substance that is a hydrogen ion donor | b. | a substance that
ionizes to yield protons in aqueous solution | c. | a substance that accepts an electron
pair | d. | a substance that is a hydrogen ion acceptor |
|
|
|
25.
|
What is a property of a base?
a. | watery feel | c. | strong color | b. | bitter taste | d. | unreactive |
|
|
|
26.
|
Which of the following represents a Brønsted-Lowry conjugate acid-base
pair?
|
|
|
27.
|
What is pH?
a. | the negative logarithm of the hydroxide ion concentration | b. | the positive
logarithm of the hydrogen ion concentration | c. | the positive logarithm of the hydroxide ion
concentration | d. | the negative logarithm of the hydrogen ion
concentration |
|
|
|
28.
|
The products of self-ionization of water are ____.
|
|
|
29.
|
What is the formula for phosphoric acid?
|
Short Answer (Value 5)
|
|
|
30.
|
A liter of impure water has 10 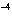 mol of hydroxide ions. What is
the concentration of hydronium ions in this sample of water?
|
|
|
31.
|
If the hydrogen-ion concentration is 1 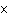 10 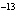 M M, what is
the pOH of the solution?
|
|
|
32.
|
What is the ion-product constant for water?
|
Numeric Response (Value 10)
|
|
|
33.
|
If [OH 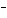 ] = 1 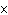 10 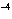 M M, what is
the pH of the solution?
|
|
|
34.
|
What is the pH when the hydrogen ion concentration is 7.0 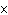 10 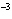 M M?
|
|
|
35.
|
If the hydroxide ion concentration is 10 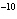 M M, what is
the pH of the solution?
|
|
|
36.
|
What is the pH of a solution with a concentration of 0.01M hydrochloric
acid?
|
|
|
37.
|
If the hydrogen ion concentration is 10 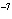 M M, what is
the pH of the solution?
|