Matching
|
|
|
Match each item with the correct statement below. a. | oxidation number | c. | oxidizing agent | b. | half-reaction | d. | reducing agent |
|
|
|
1.
|
substance that accepts electrons
|
|
|
2.
|
substance that donates electrons
|
|
|
3.
|
integer related to the number of electrons under an atom's control
|
|
|
4.
|
reaction showing either the reduction or the oxidation reaction
|
|
|
Match each item with the correct statement below. a. | Choose coefficients to make the change in oxidation number equal to
0. | b. | Make the electron changes of both half-reactions equal. | c. | Assign oxidation
numbers to all the atoms. | d. | Write the equation showing ions
separately. |
|
|
|
5.
|
the first step in balancing a redox reaction by the oxidation-number-change
method
|
|
|
6.
|
the next-to-the-last step in balancing a redox reaction by the
oxidation-number-change method
|
|
|
7.
|
the first step in balancing a redox reaction by the half-reaction
method
|
|
|
8.
|
the next-to-the-last step in balancing a redox reaction by the half-reaction
method
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
9.
|
If an atom is reduced in a redox reaction, what must happen to another atom in
the system?
a. | It must be oxidized. | b. | It must be reduced. | c. | It must be
neutralized. | d. | Nothing needs to happen to another atom in the
system. |
|
|
|
10.
|
What is another name for an oxidation-reduction reaction?
a. | O-reaction | c. | redox reaction | b. | R-reaction | d. | oxred reaction |
|
|
|
11.
|
When iron oxide becomes iron, what type of reaction occurs?
a. | oxidation | c. | neutralization | b. | reduction | d. | combination |
|
|
|
12.
|
In which of the following types of reaction are electrons gained?
a. | decomposition | c. | neutralization | b. | oxidation | d. | reduction |
|
|
|
13.
|
Oxidation is ____.
a. | a loss of oxygen | c. | a loss of electrons | b. | a gain of electrons | d. | a gain of
hydrogen |
|
|
|
14.
|
What are transferred in an oxidation-reduction reaction?
a. | protons | c. | electrons | b. | ions | d. | atoms |
|
|
|
15.
|
In the reaction of sodium with oxygen, which atom is reduced?
a. | sodium | c. | both a and b | b. | oxygen | d. | neither a nor b |
|
|
|
16.
|
In the reaction of sodium with oxygen, which atom is the reducing agent?
a. | sodium | c. | both a and b | b. | oxygen | d. | neither a nor b |
|
|
|
17.
|
In the reaction of calcium with chlorine, which atom is the oxidizing
agent?
a. | calcium | c. | both a and b | b. | chlorine | d. | neither a nor b |
|
|
|
18.
|
In the reaction of calcium with chlorine, which atom is oxidized?
a. | calcium | c. | both a and b | b. | chlorine | d. | neither a nor b |
|
|
|
19.
|
In the reaction of hydrogen with iodine, which atom is oxidized?
a. | hydrogen | c. | both a and b | b. | iodine | d. | neither a nor b |
|
|
|
20.
|
In the reaction of hydrogen with iodine, which atom is the reducing
agent?
a. | hydrogen | c. | both a and b | b. | iodine | d. | neither a nor b |
|
|
|
21.
|
Cu  ® ® Cu 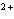 + 2 e- The equation above represents the type of reaction called ____. a. | redox | c. | reduction | b. | hydrolysis | d. | oxidation |
|
|
|
22.
|
Which type of reaction does Sn 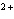 ® ®
Sn 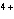 represent? a. | oxidation | c. | hydrolysis | b. | reduction | d. | none of the
above |
|
|
|
23.
|
What is the reducing agent in the following reaction? 2Na + 2H 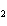 O ® 2NaOH + H 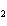 a. | Na | c. | NaOH | b. | H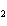 O O | d. | H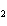 |
|
|
|
24.
|
What is the reducing agent in the following reaction? 2Na + S ® Na 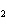 S a. | Na | c. | Na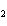 S S | b. | S | d. | Na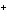 |
|
|
|
25.
|
What is the oxidizing agent in the following reaction? CH 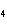 +
2O 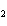 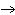 CO 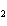 + H 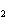 O
|
|
|
26.
|
Which statement is true about the following reaction? S + Cl 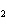 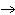 SCl 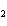 (Hint: Chlorine is the more
electronegative element.) a. | Sulfur is reduced to SCl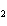 . . | c. | Chlorine is oxidized to SCl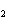 . . | b. | Chlorine is reduced to SCl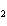 . . | d. | Sulfur is the oxidizing
agent. |
|
|
|
27.
|
What is the oxidizing agent in the following reaction? I 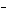 +
MnO 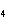 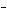 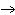 I 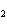 + MnO 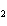
|
|
|
28.
|
Why is oxygen reduced in the reaction of hydrogen with oxygen to make
water?
a. | Oxygen pulls electrons toward itself. | b. | Oxygen pushes electrons toward the
hydrogens. | c. | Oxygen absorbs a proton. | d. | Oxygen releases a
proton. |
|
|
|
29.
|
The oxidation number of magnesium in magnesium chloride is ____.
|
|
|
30.
|
The oxidation number of hydrogen when it is in a compound other than a hydride
is ____.
|
|
|
31.
|
The oxidation number of bromime in bromime gas is ____.
|
|
|
32.
|
What is the oxidation number for each atom in NH 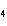 Cl? a. | N = +3, H = –4, Cl = +1 | c. | N = +3, H = +1, Cl =
–1 | b. | N = –3, H = +1, Cl = –1 | d. | N = –3, H = –1, Cl =
+1 |
|
|
|
33.
|
What is defined as the charge an atom would have in a compound if its bonding
electrons were assigned to the more electronegative atom?
a. | reduction number | c. | valence | b. | oxidation number | d. | electropositivity |
|
|
|
34.
|
The oxidation number of sulfur in each of the following is +6 EXCEPT for
____.
|
|
|
35.
|
In which of the following compounds is the oxidation number of nitrogen
different from the other three?
|
|
|
36.
|
Identify the atom that increases in oxidation number in the following redox
reaction. 2MnO 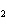 + 2K 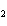 CO 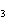 + O 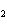 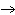 2KMnO 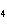 + 2CO 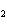
|
|
|
37.
|
Which atom has a change in oxidation number of –3 in the following redox
reaction? K 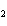 Cr 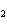 O 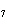 + H 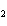 O + S
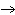 KOH + Cr 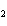 O 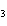 +
SO 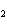
|
|
|
38.
|
In the following unbalanced reaction, which atom is reduced? H 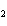 O +
Cl 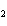 + SO 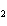  HCl + H 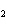 SO 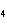 a. | hydrogen | c. | chlorine | b. | oxygen | d. | sulfur |
|
|
|
39.
|
In the following unbalanced reaction, which atom is oxidized? HNO 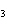 + HBr  NO + Br 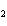 + H 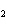 O a. | hydrogen | c. | oxygen | b. | nitrogen | d. | bromine |
|
|
|
40.
|
Which element decreases its oxidation number in the following
reaction? BiCl 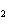 + Na 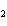 SO 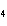 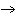 2NaCl + BiSO 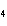 a. | bismuth | b. | chlorine | c. | oxygen | d. | No element decreases its oxidation
number. |
|
|
|
41.
|
Which element increases its oxidation number in the following reaction? 3KOH
+ H 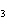 PO 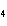 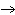 K 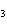 PO 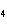 + 3H 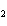 O a. | potassium | b. | hydrogen | c. | oxygen | d. | No element increases its oxidation
number. |
|
|
|
42.
|
Which element decreases its oxidation number in the following reaction? I 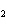 + 2KCl 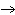 2KI + Cl 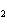 a. | iodine | b. | potassium | c. | chlorine | d. | No element decreases its oxidation
number. |
|
|
|
43.
|
Which element increases its oxidation number in the following reaction? 2Na +
2H 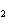 O 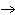 2NaOH + H 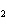 a. | sodium | b. | hydrogen | c. | oxygen | d. | No element increases its oxidation
number. |
|
|
|
44.
|
Which of the following reactions is a redox reaction?
a. | acid-base | c. | combustion | b. | double-replacement | d. | all of the
above |
|
Numeric Response
|
|
|
45.
|
What is the oxidation number of magnesium in magnesium iodide?
|
|
|
46.
|
What is the sum of the oxidation numbers in lithium carbonate?
|
|
|
47.
|
What is the sum of the oxidation numbers in calcium carbonate?
|
|
|
48.
|
What is the sum of the oxidation numbers in the chlorate ion?
|