Matching
|
|
|
Match each item with the correct statement below. a. | monatomic ion | f. | cation | b. | acid | g. | binary compound | c. | base | h. | anion | d. | law of definite proportions | i. | polyatomic ion | e. | law of multiple
proportions |
|
|
|
1.
|
consists of a single atom with a positive or negative charge
|
|
|
2.
|
atom or group of atoms having a negative charge
|
|
|
3.
|
atom or group of atoms having a positive charge
|
|
|
4.
|
tightly-bound group of atoms that behaves as a unit and carries a net
charge
|
|
|
5.
|
produces a hydroxide ion when dissolved in water
|
|
|
6.
|
In any chemical compound, the masses of elements are always in the same
proportion by mass.
|
|
|
7.
|
when two elements form more than one compound, the masses of one element that
combine with the same mass of the other element are in the ratio of small, whole numbers
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
8.
|
What type of ions have names ending in -ide?
a. | only cations | c. | only metal ions | b. | only anions | d. | only gaseous
ions |
|
|
|
9.
|
Which of the following correctly provides the name of the element, the symbol
for the ion, and the name of the ion?
a. | fluorine, F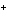 , fluoride ion , fluoride ion | c. | copper, Cu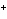 , cuprous ion , cuprous ion | b. | zinc, Zn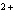 , zincate
ion , zincate
ion | d. | sulfur, S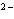 , sulfurous ion , sulfurous ion |
|
|
|
10.
|
The nonmetals in Groups 6A and 7A ____.
a. | lose electrons when they form ions | b. | have a numerical charge that is found by
subtracting 8 from the group number | c. | all have ions with a –1
charge | d. | end in -ate |
|
|
|
11.
|
What determines that an element is a metal?
a. | the magnitude of its charge | c. | when it is a Group A
element | b. | the molecules that it forms | d. | its position in the periodic table |
|
|
|
12.
|
Which of the following is NOT a cation?
a. | iron(III) ion | c. | Ca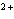 | b. | sulfate | d. | mercurous ion |
|
|
|
13.
|
In which of the following are the symbol and name for the ion given
correctly?
|
|
|
14.
|
Why are systematic names preferred over common names?
a. | Common names do not provide information about the chemical composition of the
compound. | b. | Common names are derived from the method used to obtain the
compound. | c. | Common names were assigned by the scientist who discovered the
compound. | d. | Common names are not very descriptive. |
|
|
|
15.
|
How are chemical formulas of binary ionic compounds generally written?
a. | cation on left, anion on right | b. | anion on left, cation on
right | c. | Roman numeral first, then anion, then cation | d. | subscripts first,
then ions |
|
|
|
16.
|
Which of the following formulas represents an ionic compound?
|
|
|
17.
|
In which of the following are the formula of the ionic compound and the charge
on the metal ion shown correctly?
|
|
|
18.
|
Which set of chemical name and chemical formula for the same compound is
correct?
a. | ammonium sulfite, (NH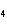 ) )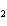 S S | c. | lithium carbonate, LiCO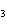 | b. | iron(III) phosphate,
FePO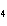 | d. | magnesium dichromate, MgCrO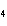 |
|
|
|
19.
|
What type of compound is CuSO 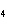 ? a. | monotomic ionic | c. | polyatomic ionic | b. | polyatomic covalent | d. | binary
molecular |
|
|
|
20.
|
Which polyatomic ion forms a neutral compound when combined with a group 1A
monatomic ion in a 1:1 ratio?
a. | ammonium | c. | nitrate | b. | carbonate | d. | phosphate |
|
|
|
21.
|
Molecular compounds are usually ____.
a. | composed of two or more transition elements | b. | composed of positive
and negative ions | c. | composed of two or more nonmetallic
elements | d. | exceptions to the law of definite proportions |
|
|
|
22.
|
What is the formula for hydrosulfuric acid?
|
|
|
23.
|
Which of the following are produced when a base is dissolved in water?
a. | hydronium ions | c. | hydrogen ions | b. | hydroxide ions | d. | ammonium ions |
|
|
|
24.
|
What is the correct name for Sn 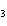 (PO 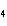 ) 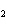 ? a. | tritin diphosphate | c. | tin(III) phosphate | b. | tin(II) phosphate | d. | tin(IV)
phosphate |
|
|
|
25.
|
How many hydrogen atoms are in 5 molecules of isopropyl alcohol, C 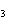 H 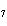 O? a. | 5 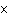 (6.02 (6.02 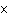 10 10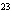 ) ) | c. | 35 | b. | 5 | d. | 35 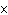 (6.02 (6.02 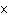 10 10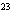 ) ) |
|
|
|
26.
|
How many atoms are in 0.075 mol of titanium?
|
|
|
27.
|
What is the molar mass of (NH 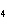 ) 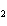 CO 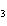 ? a. | 144 g | c. | 96 g | b. | 138 g | d. | 78 g |
|
|
|
28.
|
How many moles of CaBr 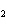 are in 5.0 grams of
CaBr 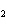 ?
|
|
|
29.
|
For which of the following conversions does the value of the conversion factor
depend upon the formula of the substance?
a. | volume of gas (STP) to moles | b. | density of gas (STP) to molar
mass | c. | mass of any substance to moles | d. | moles of any substance to number of
particles |
|
|
|
30.
|
What is the mass of silver in 3.4 g AgNO 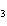 ? a. | 0.025 g | c. | 2.2 g | b. | 0.64 g | d. | 3.0 g |
|
|
|
31.
|
If the density of an unknown gas Z is 4.50 g/L at STP, what is the molar mass of
gas Z?
a. | 0.201 g/mol | c. | 26.9 g/mol | b. | 5.00 g/mol | d. | 101 g/mol |
|
|
|
32.
|
To determine the formula of a new substance, one of the first steps is to find
the ____.
a. | molar mass | c. | volume at STP | b. | percent composition | d. | number of particles per
mole |
|
|
|
33.
|
What information is needed to calculate the percent composition of a
compound?
a. | the weight of the sample to be analyzed and its density | b. | the weight of the
sample to be analyzed and its molar volume | c. | the formula of the compound and the atomic mass
of its elements | d. | the formula of the compound and its density |
|
|
|
34.
|
If 60.2 grams of Hg combines completely with 24.0 grams of Br to form a
compound, what is the percent composition of Hg in the compound?
a. | 28.5% | c. | 71.5% | b. | 39.9% | d. | 60.1% |
|
|
|
35.
|
What is the percent composition of chromium in BaCrO 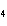 ? a. | 4.87% | c. | 20.5% | b. | 9.47% | d. | 25.2% |
|
|
|
36.
|
If 20.0 grams of Ca combines completely with 16.0 grams of S to form a compound,
what is the percent composition of Ca in the compound?
a. | 1.25% | c. | 44.4% | b. | 20.0% | d. | 55.6% |
|
|
|
37.
|
Which of the following compounds has the highest oxygen content, by
weight?
|
|
|
38.
|
Which of the following is NOT an empirical formula?
|
|
|
39.
|
What is the empirical formula of a compound that is 40% sulfur and 60% oxygen by
weight?
|
|
|
40.
|
The ratio of carbon atoms to hydrogen atoms to oxygen atoms in a molecule of
dicyclohexyl maleate is 4 to 6 to 1. What is its molecular formula if its molar mass is 280 g?
|
|
|
41.
|
Everyday equations describe ____.
a. | thermonuclear reactions | c. | chemical
reactions | b. | everyday processes | d. | biological chemistry |
|
|
|
42.
|
Chemical equations ____.
a. | describe chemical reactions | b. | show how to write chemical
formulas | c. | give directions for naming chemical compounds | d. | describe only
biological changes |
|
|
|
43.
|
A skeleton equation does NOT show which of the following?
a. | the correct formulas of the reactants and products | b. | the reactants on the
left, the products on the right | c. | an arrow connecting the reactants to the
products | d. | the relative amounts of reactants and products |
|
|
|
44.
|
Symbols used in equations, together with the explanations of the symbols, are
shown below. Which set is correct?
a. | (g), grams | c. | (aq), dissolved in water | b. | (l),
liters | d. | (s), solid
product |
|
|
|
45.
|
Which of the following is the correct skeleton equation for the reaction that
takes place when solid phosphorus combines with oxygen gas to form diphosphorus pentoxide?
|
|
|
46.
|
What are the coefficients that will balance the skeleton equation
below? AlCl  + NaOH  Al(OH)   NaCl a. | 1, 3, 1, 3 | c. | 1, 1, 1, 3 | b. | 3, 1, 3, 1 | d. | 1, 3, 3, 1 |
|
|
|
47.
|
What are the coefficients that will balance the skeleton equation below?
N  + H   NH  a. | 1, 1, 2 | c. | 3, 1, 2 | b. | 1, 3, 3 | d. | 1, 3, 2 |
|
|
|
48.
|
When the equation Fe  Cl   FeCl  is balanced, what is the coefficient for
Cl  ?
|
|
|
49.
|
What are the missing coefficients for the skeleton equation
below? Cr( s)  Fe(NO  )  ( aq)  Fe( s)  Cr(NO  )  ( aq) a. | 4, 6, 6, 2 | c. | 2, 3, 3, 2 | b. | 2, 3, 2, 3 | d. | 1, 3, 3, 1 |
|
|
|
50.
|
Use the activity series of metals to complete a balanced chemical equation for
the following single replacement reaction. Ag( s)  KNO  ( aq)  a. | AgNO  K K | b. | AgK  NO
NO | c. | AgKNO | d. | No reaction takes place because silver is less
reactive than potassium. |
|
|
|
51.
|
Which of the following statements is NOT true about double-replacement
reactions?
a. | The product may precipitate from solution. | b. | The product may be a
gas. | c. | The product may be a molecular compound. | d. | The reactant may be
a solid metal. |
|
|
|
52.
|
Which of the following is the correctly balanced equation for the incomplete
combustion of heptene, C  H  ?
|
|
|
53.
|
The reaction 2Fe  3Cl   2FeCl  is an example of which type of
reaction? a. | combustion reaction | c. | combination reaction | b. | single-replacement reaction | d. | decomposition
reaction |
|
|
|
54.
|
The equation 2C  H  OH  9O   6CO   8H  O is an example of which type of reaction? a. | combustion reaction | c. | double-replacement reaction | b. | single-replacement
reaction | d. | decomposition
reaction |
|
|
|
55.
|
What is the driving force in the following reaction? Ni(NO  )  ( aq)  K  S( aq)  NiS (s)  2KNO  ( aq) a. | A gas is formed. | c. | Ionic compounds are reactants. | b. | A precipitate is
formed. | d. | Ionic compounds are
products. |
|
|
|
56.
|
What is conserved in the reaction shown below? H  ( g) + Cl  ( g) ®
2HCl( g) a. | mass only | c. | mass, moles, and molecules only | b. | mass and moles
only | d. | mass, moles,
molecules, and volume |
|
|
|
57.
|
In the reaction 2CO( g) + O  ( g) ® 2CO  ( g), what is the ratio of moles of
oxygen used to moles of CO  produced?
|
|
|
58.
|
Hydrogen gas can be produced by reacting aluminum with sulfuric acid. How many
moles of sulfuric acid are needed to completely react with 15.0 mol of aluminum? 2Al( s) +
3H  SO  ( aq)
® Al  (SO  )  ( aq) + 3H  ( g) a. | 0.100 mol | c. | 15.0 mol | b. | 10.0 mol | d. | 22.5 mol |
|
|
|
59.
|
When iron rusts in air, iron(III) oxide is produced. How many moles of oxygen
react with 2.4 mol of iron in the rusting reaction? 4Fe( s) + 3O  ( g) ® 2Fe2O  ( s) a. | 1.2 mol | c. | 2.4 mol | b. | 1.8 mol | d. | 3.2 mol |
|
|
|
60.
|
Iron(III) oxide is formed when iron combines with oxygen in the air. How many
grams of Fe  O  are formed
when 16.7 g of Fe reacts completely with oxygen?  a. | 12.0 g | c. | 47.8 g | b. | 23.9 g | d. | 95.6 g |
|
|
|
61.
|
When glucose is consumed, it reacts with oxygen in the body to produce carbon
dioxide, water, and energy. How many grams of carbon dioxide would be produced if 45 g of C  H  O  completely
reacted with oxygen? a. | 1.5 g | c. | 11 g | b. | 1.8 g | d. | 66 g |
|
|
|
62.
|
How many liters of hydrogen gas are needed to react with CS 
to produce 2.50 L of CH  at STP?  a. | 2.50 L | c. | 7.50 L | b. | 5.00 L | d. | 10.0 L |
|
|
|
63.
|
Which of the following statements is true about the following
reaction? 3NaHCO  ( aq) + C  H  O  ( aq)
 3CO  ( g) +
3H  O( s) +Na  C  H  O  ( aq)
|