Multiple Choice (Value 60)( Best of 60)
Identify the choice that best completes the statement or answers
the question.
|
|
|
1.
|
An example of an extensive property of matter is ____.
a. | temperature | c. | mass | b. | pressure | d. | hardness |
|
|
|
2.
|
Which of the following are considered physical properties of a substance?
a. | color and odor | c. | malleability and hardness | b. | melting and boiling
points | d. | all of the
above |
|
|
|
3.
|
A substance that forms a vapor is generally in what physical state at room
temperature?
a. | solid | c. | gas | b. | liquid | d. | liquid or solid |
|
|
|
4.
|
Which state of matter takes both the shape and volume of its container?
a. | solid | c. | gas | b. | liquid | d. | both b and c |
|
|
|
5.
|
Which state of matter is characterized by having a definite shape and a definite
volume?
a. | gas | c. | solid | b. | liquid | d. | all of the
above |
|
|
|
6.
|
Which state of matter expands when heated and is easy to compress?
a. | gas | c. | solid | b. | liquid | d. | all of the
above |
|
|
|
7.
|
Which of the following CANNOT be classified as a substance?
a. | table salt | c. | nitrogen | b. | air | d. | gold |
|
|
|
8.
|
Which of the following is a heterogeneous mixture?
a. | air | c. | steel | b. | salt water | d. | soil |
|
|
|
9.
|
An example of a homogeneous mixture is ____.
a. | water | c. | noodle soup | b. | stainless steel | d. | oxygen |
|
|
|
10.
|
Which of the following items is NOT a compound?
a. | baking soda | c. | sucrose | b. | salad dressing | d. | table salt |
|
|
|
11.
|
What distinguishes a substance from a mixture?
a. | Substances are compounds, and mixtures are not. | b. | Mixtures are
groupings of elements, and compounds are not. | c. | Samples of the same substance can have
different intensive properties. | d. | Mixtures can be separated physically, while
compounds cannot. |
|
|
|
12.
|
What do chemical symbols and formulas represent, respectively?
a. | elements and compounds | b. | atoms and mixtures | c. | compounds and
mixtures | d. | elements and ions |
|
|
|
13.
|
Which of the following is a chemical property?
a. | color | c. | freezing point | b. | hardness | d. | ability to react with
oxygen |
|
|
|
14.
|
Which of the following does NOT indicate that a chemical change may have taken
place?
a. | fracture formation | c. | precipitate formation | b. | gas
production | d. | energy
transfer |
|
|
|
15.
|
What happens to matter during a chemical reaction?
a. | Matter is neither destroyed or created. | b. | Some matter is
destroyed. | c. | Some matter is created. | d. | Some matter is destroyed and some is
created. |
|
|
|
16.
|
Who was the man who lived from 460B.C.–370B.C. and was among the first to
suggest the idea of atoms?
a. | Atomos | c. | Democritus | b. | Dalton | d. | Thomson |
|
|
|
17.
|
Which of the following was NOT among Democritus’s ideas?
a. | Matter consists of tiny particles called atoms. | b. | Atoms are
indivisible. | c. | Atoms retain their identity in a chemical reaction. | d. | Atoms are
indestructible. |
|
|
|
18.
|
Dalton's atomic theory included which idea?
a. | All atoms of all elements are the same size. | b. | Atoms of different
elements always combine in one-to-one ratios. | c. | Atoms of the same element are always
identical. | d. | Individual atoms can be seen with a microscope. |
|
|
|
19.
|
Which of the following was originally a tenet of Dalton's atomic theory,
but had to be revised about a century ago?
a. | Atoms are tiny indivisible particles. | b. | Atoms of the same element are
identical. | c. | Compounds are made by combining atoms. | d. | Atoms of different elements can combine with
one another in simple whole number ratios. |
|
|
|
20.
|
The range in size of most atomic radii is approximately ____.
|
|
|
21.
|
Which of the following is true about subatomic particles?
a. | Electrons are negatively charged and are the heaviest subatomic
particle. | b. | Protons are positively charged and the lightest subatomic
particle. | c. | Neutrons have no charge and are the lightest subatomic particle. | d. | The mass of a
neutron nearly equals the mass of a proton. |
|
|
|
22.
|
Who conducted experiments to determine the quantity of charge carried by an
electron?
a. | Rutherford | c. | Dalton | b. | Millikan | d. | Thomson |
|
|
|
23.
|
What is the relative mass of an electron?
a. | 1/1840 the mass of a hydrogen atom | c. | 1/1840 the mass of a C-12
atom | b. | 1/1840 the mass of a neutron + proton | d. | 1/1840 the mass of an alpha
particle |
|
|
|
24.
|
Which of the following is correct concerning subatomic particles?
a. | The electron was discovered by Goldstein in 1886. | b. | The neutron was
discovered by Chadwick in 1932. | c. | The proton was discovered by Thomson in
1880. | d. | Cathode rays were found to be made of protons. |
|
|
|
25.
|
The nucleus of an atom is ____.
a. | the central core and is composed of protons and neutrons | b. | positively charged
and has more protons than neutrons | c. | negatively charged and has a high
density | d. | negatively charged and has a low density |
|
|
|
26.
|
The atomic number of an element is the total number of which particles in the
nucleus?
a. | neutrons | c. | electrons | b. | protons | d. | protons and
electrons |
|
|
|
27.
|
Isotopes of the same element have different ____.
a. | positions on the periodic table | c. | atomic numbers | b. | chemical
behavior | d. | mass
numbers |
|
|
|
28.
|
Which of the following statements is NOT true?
a. | Atoms of the same element can have different masses. | b. | Atoms of isotopes of
an element have different numbers of protons. | c. | The nucleus of an atom has a positive
charge. | d. | Atoms are mostly empty space. |
|
|
|
29.
|
Which of the following statements is NOT true?
a. | Protons have a positive charge. | b. | Electrons are negatively charged and have a
mass of 1 amu. | c. | The nucleus of an atom is positively charged. | d. | Neutrons are located
in the nucleus of an atom. |
|
|
|
30.
|
In Bohr's model of the atom, where are the electrons and protons
located?
a. | The electrons move around the protons, which are at the center of the
atom. | b. | The electrons and protons move throughout the atom. | c. | The electrons occupy
fixed positions around the protons, which are at the center of the atom. | d. | The electrons and
protons are located throughout the atom, but they are not free to
move. |
|
|
|
31.
|
What is the shape of the 3p atomic orbital?
a. | sphere | c. | bar | b. | dumbbell | d. | two perpendicular
dumbbells |
|
|
|
32.
|
How many energy sublevels are in the second principal energy level?
|
|
|
33.
|
What is the maximum number of orbitals in the p sublevel?
|
|
|
34.
|
What is the maximum number of electrons in the second principal energy
level?
|
|
|
35.
|
When an electron moves from a lower to a higher energy level, the electron
____.
a. | always doubles its energy | b. | absorbs a continuously variable amount of
energy | c. | absorbs a quantum of energy | d. | moves closer to the
nucleus |
|
|
|
36.
|
If the spin of one electron in an orbital is clockwise, what is the spin of the
other electron in that orbital?
a. | zero | c. | counterclockwise | b. | clockwise | d. | both clockwise and
counterclockwise |
|
|
|
37.
|
What is the next atomic orbital in the series 1s, 2s, 2p,
3s, 3p?
|
|
|
38.
|
What is the number of electrons in the outermost energy level of an oxygen
atom?
|
|
|
39.
|
How many unpaired electrons are in a sulfur atom (atomic number 16)?
|
|
|
40.
|
Stable electron configurations are likely to contain ____.
a. | filled energy sublevels | b. | fewer electrons than unstable
configurations | c. | unfilled s orbitals | d. | electrons with a clockwise
spin |
|
|
|
41.
|
Which of the following electron configurations of outer sublevels is the most
stable?
|
|
|
42.
|
What is the approximate energy of a photon having a frequency of 4 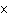
10 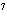 Hz? ( h = 6.6 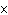 10 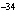 J  s)
|
|
|
43.
|
What is another name for the representative elements?
a. | Group A elements | c. | Group C elements | b. | Group B elements | d. | transition
elements |
|
|
|
44.
|
What is another name for the transition metals?
a. | noble gases | c. | Group B elements | b. | Group A elements | d. | Group C
elements |
|
|
|
45.
|
Of the elements Pt, V, Li, and Kr, which is a nonmetal?
|
|
|
46.
|
In which of the following sets is the symbol of the element, the number of
protons, and the number of electrons given correctly?
a. | In, 49 protons, 49 electrons | c. | Cs, 55 protons, 132.9
electrons | b. | Zn, 30 protons, 60 electrons | d. | F, 19 protons, 19
electrons |
|
|
|
47.
|
Which of the following is true about the electron configurations of the noble
gases?
a. | The highest occupied s and p sublevels are completely
filled. | b. | The highest occupied s and p sublevels are partially
filled. | c. | The electrons with the highest energy are in a d sublevel. | d. | The electrons with
the highest energy are in an f sublevel. |
|
|
|
48.
|
Which of the following elements is a transition metal?
a. | cesium | c. | tellurium | b. | copper | d. | tin |
|
|
|
49.
|
Which of the following groupings contains only representative elements?
a. | Cu, Co, Cd | c. | Al, Mg, Li | b. | Ni, Fe, Zn | d. | Hg, Cr, Ag |
|
|
|
50.
|
Which of the following is true about the electron configurations of the
representative elements?
a. | The highest occupied s and p sublevels are completely
filled. | b. | The highest occupied s and p sublevels are partially
filled. | c. | The electrons with the highest energy are in a d sublevel. | d. | The electrons with
the highest energy are in an f sublevel. |
|
|
|
51.
|
What are the Group 1A and Group 7A elements examples of?
a. | representative elements | c. | noble gases | b. | transition
elements | d. | nonmetallic
elements |
|
|
|
52.
|
How does atomic radius change from top to bottom in a group in the periodic
table?
a. | It tends to decrease. | c. | It first increases, then decreases. | b. | It tends to
increase. | d. | It first
decreases, then increases. |
|
|
|
53.
|
What element in the second period has the largest atomic radius?
a. | carbon | c. | potassium | b. | lithium | d. | neon |
|
|
|
54.
|
What is the charge of a cation?
a. | a positive charge | b. | no charge | c. | a negative
charge | d. | The charge depends on the size of the nucleus. |
|
|
|
55.
|
Which of the following statements is true about ions?
a. | Cations form when an atom gains electrons. | b. | Cations form when an
atom loses electrons. | c. | Anions form when an atom gains
protons. | d. | Anions form when an atom loses protons. |
|
|
|
56.
|
Which of the following statements is NOT true about ions?
a. | Cations are positively charged ions. | b. | Anions are common among
nonmetals. | c. | Charges for ions are written as numbers followed by a plus or minus
sign. | d. | When a cation forms, more electrons are transferred to
it. |
|
|
|
57.
|
In which of the following sets are the charges given correctly for all the
ions?
|
|
|
58.
|
Which of the following elements has the smallest first ionization energy?
a. | sodium | c. | potassium | b. | calcium | d. | magnesium |
|
|
|
59.
|
As you move from left to right across the second period of the periodic table
____.
a. | ionization energy increases | c. | electronegativity
decreases | b. | atomic radii increase | d. | atomic mass decreases |
|
|
|
60.
|
The noble gases are the family of elements in
group
|
|
|
61.
|
What does STP stand for
a. | Standard Temperature and Pressure | c. | Stinky toilet
paper | b. | Specific Temperature and Pressure | d. | Ambient Temperature and
Pressure |
|
|
|
62.
|
The difference in mass of isotopes of the same element is due to different
numbers of _____ in the nucleus
a. | Electron | c. | Neutron | b. | Ptroton | d. | Quark |
|
Numeric Response (Answer 2 of 3)(Value 2)
|
|
|
63.
|
Determine the number of electrons in an atom of iridium.
|
|
|
64.
|
How many electrons are in the highest occupied energy level of a neutral
strontium atom?
|
|
|
65.
|
How many electrons does the ion Ca 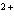 contain?
|
Complete the below table (Value 21)
|
|
|
66.
|
Isotope Name | Atomic Number | Mass Number | Standard Atomic
Notation | # of Protons | #
of Neutrons | Phosphorous-35 | | | 1535P | | | | | | 140 | 56140Ba | 56 | | | | 27 | 60 | 2760Co | | | | | 92 | | 92238U | | 146 | Uranium-235 | | | 92235U | | | | | | | | |
|